Abstract
Background/Introduction: Despite overall improvements in outcomes of diffuse large B-cell lymphoma (DLBCL), about one-third of patients will develop relapsed/refractory (R/R) disease, which remains a major cause of mortality. Effective therapies for R/R DLBCL are limited in China, especially for those patients not eligible for high-dose therapy/stem cell transplantation (HDT/SCT). Zanubrutinib, a potent and selective inhibitor of Bruton tyrosine kinase, is approved for various B-cell malignancies, and preclinical data suggest potential synergy when combined with lenalidomide. Here, we present the preliminary results from the dose-escalation part of an ongoing phase 1, open-label, multicenter, dose-escalation and -expansion study of zanubrutinib plus lenalidomide combination treatment in patients with R/R DLBCL (NCT04436107).
Methods: Patients with R/R DLBCL who had received at least 1 prior line of adequate systemic therapy and were ineligible for HDT/SCT were enrolled. In the dose-escalation part, patients received lenalidomide at 15 mg (dose level 1), 20 mg (dose level 2), or 25 mg (dose level 3) orally once daily on days 1-21 of each 28-day cycle. All dose levels were given in combination with zanubrutinib 160 mg orally twice daily continuously until progressive disease or intolerable toxicity. The primary endpoints of the dose-escalation part were safety and the recommended phase 2 dose (RP2D) of lenalidomide in the combination.
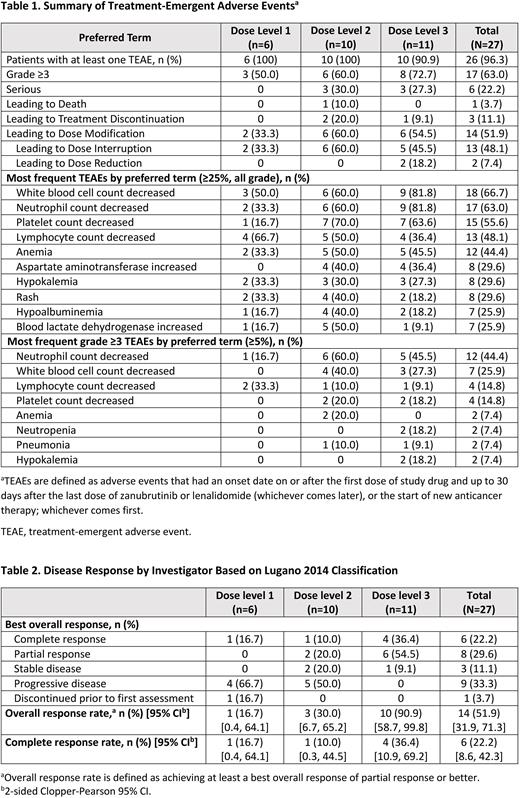
Results: As of June 6, 2022, a total of 27 patients were enrolled and treated in the dose-escalation part (6 at dose level 1, 10 at dose level 2, and 11 at dose level 3). Median age was 58.0 years (range 29-77), 74.1% of patients had stage III-IV disease, 51.9% had refractory disease, and 63.0% had non-germinal center B-cell like disease. Median number of prior systemic therapies was 1 (range 1-5). Median treatment exposure was 5.6 months (range 0.4-19.8) for both zanubrutinib and lenalidomide and the median follow-up was 8.3 months (range 0.5-20.4). Twenty-six (96.3%) patients experienced at least 1 treatment-emergent adverse event (TEAE), and the incidence of any grade TEAEs did not increase significantly with increasing dose. Grade ≥3 TEAEs occurred in 63.0% of patients. The most common grade ≥3 TEAEs were hematologic toxicities and were generally manageable across all dose levels. No patients experienced dose-limiting toxicity at any tested dose level. Maximum tolerated dose was not reached. One TEAE leading to death was reported at dose level 2, and was assessed as not related to the study treatment. The summary of TEAEs is listed in Table 1.
The overall response rate (ORR) was 51.9%, with the best ORR at dose level 3 (90.9%; Table 2). The overall complete response (CR) rate was 22.2%, and patients treated with dose level 3 displayed the highest CR rate at 36.4%. The median duration of response was not reached across all dose levels (overall 95% CI: 3.02-not estimable). Based on the strong efficacy and manageable safety demonstrated, the RP2D of lenalidomide in the combination was determined to be dose level 3.
Conclusion: Zanubrutinib and lenalidomide combination treatment showed an acceptable safety profile and promising preliminary efficacy data in patients with R/R DLBCL, with a dose-dependent increase in response in the 3 dose cohorts. The dose-expansion part of the study, which uses dose level 3 (zanubrutinib 160 mg twice daily continuously, lenalidomide 25 mg once daily on days 1-21 of each 28-day cycle) as the RP2D, is now recruiting.
Disclosures
Huang:BeiGene (Shanghai) Co., Ltd: Current Employment, Current equity holder in publicly-traded company. Liang:BeiGene Ltd: Current Employment, Current equity holder in publicly-traded company, Other: Travel, Accommodations, Expenses. Yao:BeiGene Ltd: Current Employment, Current equity holder in publicly-traded company, Other: travel, accommodations, expenses. Guo:BeiGene Ltd: Current Employment, Current equity holder in publicly-traded company, Other: Travel, Accommodations, and Expenses. Zhu:BeiGene: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal